 Covalent bonds.
Covalent bonds.
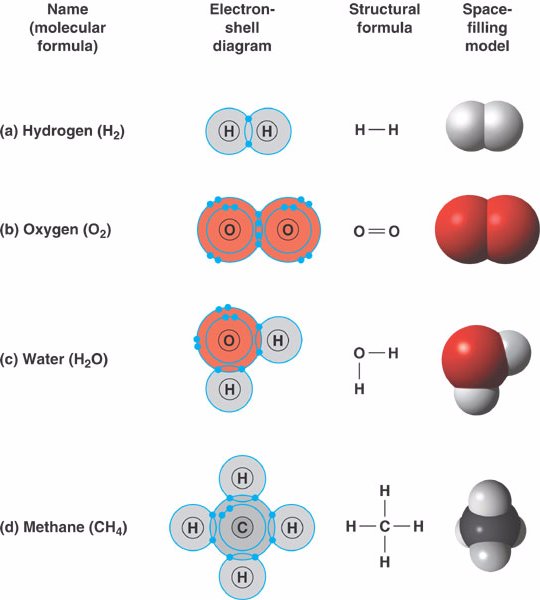
- Hydrogen (H2):
two hydrogen atoms can form a single covalent bond by sharing one pair of electrons.
- Oxygen (O2):
two oxygen atoms share two pairs of electrons to form a double covalent bond.
- Water (H2O):
two hydrogen atoms and one oxygen atom are joined by single covalent bonds to produce a molecule of water.
- Methane (CH4):
four hydrogen atoms can satisfy the valence of one carbon atom,
forming methane.
- Q: What kind of bonds form between these hydrogen atoms and carbon?
- A: Single covalent bonds.
 Covalent bonds.
Covalent bonds.