
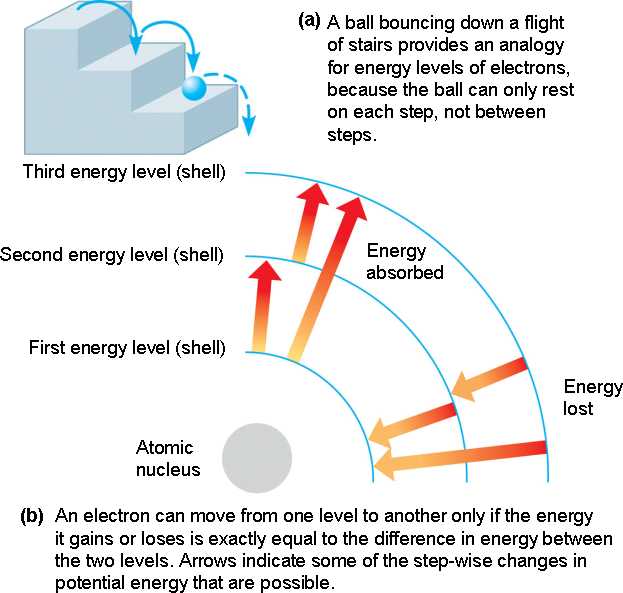
 Electron shells.
Electron shells.
Electrons exist only at fixed levels of energy, which are also called electron shells.
The outermost shell is called the valence shell, and contains valence electrons.
Most chemical reactions involve valence electrons, since atoms are most stable with complete valence shells.